Blog
Environmental Monitoring Program

More food facilities are creating and upgrading their environmental monitoring programs (EMP) due to an increasing emphasis from government agencies on locating and controlling food hazards and contaminants. The FDA Food Safety Modernization Act (FSMA) changed the nation’s food safety system by altering the focus from responding to foodborne illness to preventing it. Congress enacted FSMA as a response to the realization that foodborne illness is both a significant public health problem and a severe threat to our food system’s economic well-being.
For food manufacturers, the routine applications of good sanitation practices can control microorganisms inside the food processing and handling environments. However, if contamination levels are high or sanitation procedures are inadequate, these microorganisms may grow and contaminate food products leading to a foodborne illness outbreak.
This article covers how to build and execute an EMP, when to conduct microbial and ATP testing, and enumerates the advantages of in-house environmental testing over third-party testing.
What Does an Environmental Monitoring Program Involve?
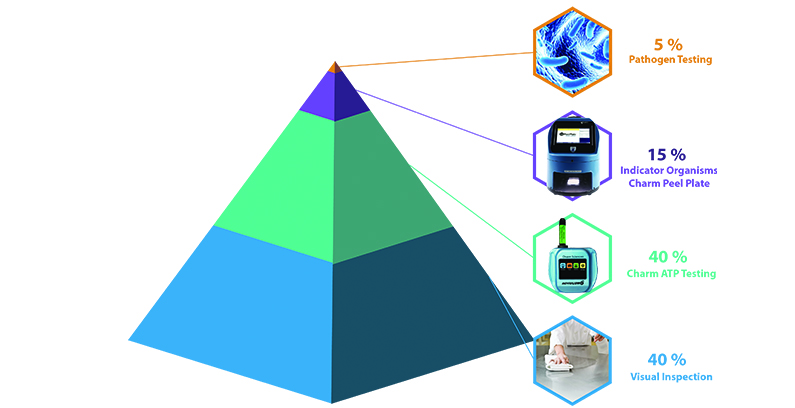
Traditionally, an EMP involves assessing the environment’s hygienic practices and the effectiveness of microbial controls. The principle building blocks of surface assessment include visual inspection, ATP testing, indicator microbial testing, and pathogen testing. In addition, it involves testing zones 1, 2, 3, and 4, outlined in more detail below. Results are then analyzed, which may prompt the initiation of appropriate corrective actions, including recleaning, resanitizing, and retraining. Finally, manufacturers document all actions and data is tracked, sometimes by use of a LIMS program.
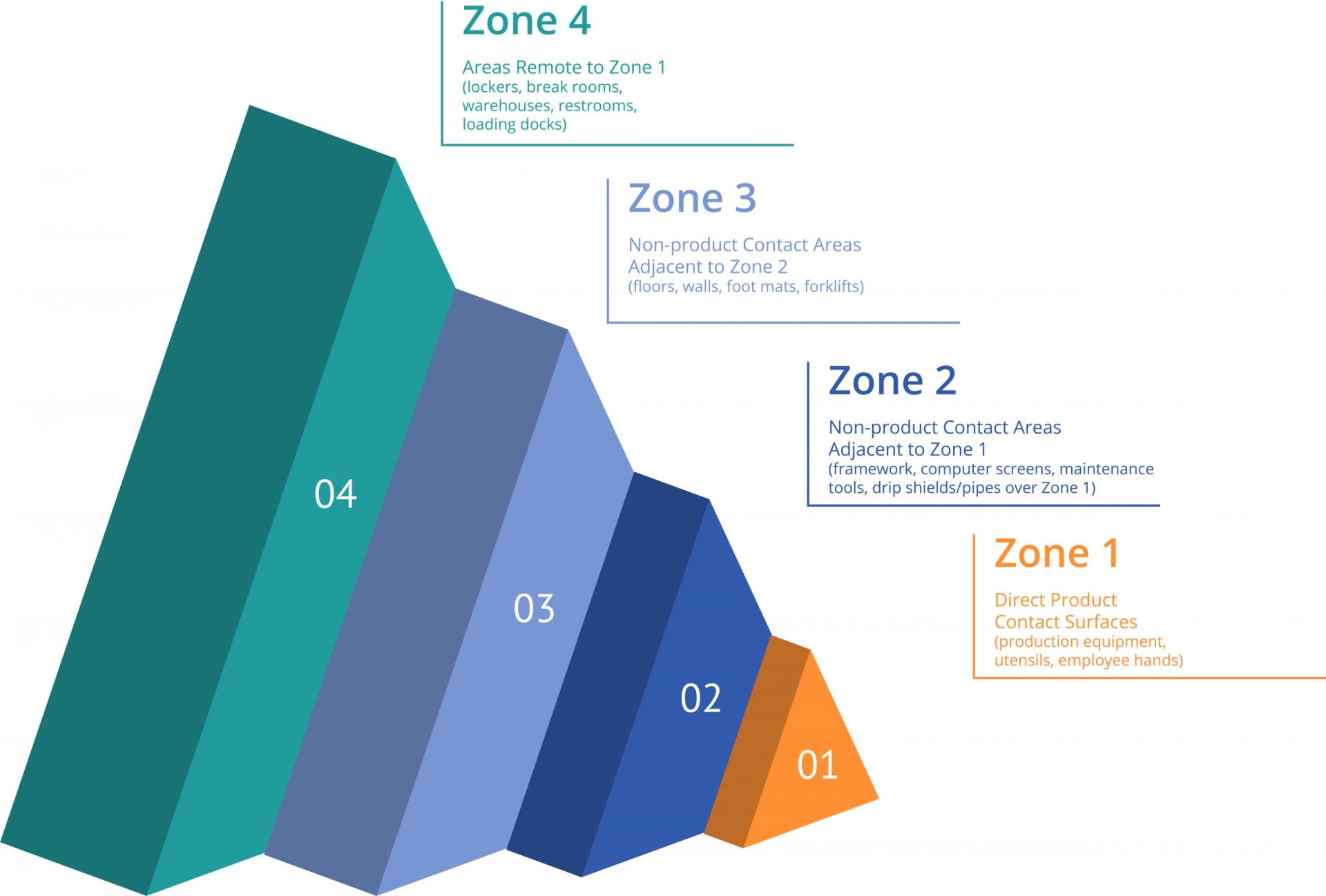
Structure food contact zones according to their proximity to food.
Zone 1: Direct product contact surfaces. Examples are conveyors/buckets, utensils, employee hands, slicers/pitters, and hoppers/bins/bin liners/fillers.
Zone 2: Non-product contact sites adjacent to Zone 1. Examples are equipment framework, drip shields/housing/control panels/buttons, pipes over Zone 1, computer screens, and maintenance tools.
Zone 3: Non-product contact sites adjacent to Zone 2. Examples are floors/walls/ceilings, hoses/air handling units, drains, foot mats/baths, forklifts, brooms/mops, and pallets.
Zone 4: Areas remote to Zone 1. Examples are locker/break rooms/offices, warehouses/freezers/cold storage, restrooms, loading docks, and maintenance shop.
An EMP bases the frequency of taking samples on regulation, risk, and industry best practices.
An EMP :
- Acts as an early warning system for microbiological hazards in both the production and post-production environment. When well-developed and effectively implemented, an EMP is an integral component of prerequisite programs.
- Helps to identify harborage niches and hot spots in a plant that may act as a source of contamination.
- Validates the sanitation program and helps in determining the frequency required for cleaning and sanitation.
When to Conduct ATP Testing?
Product residue, microbes, and all other organic substances contain adenosine triphosphate (ATP). Effective cleaning and sanitation help eliminate ATP from the food contact surfaces and food plant environment. A positive ATP test reveals an unclean surface. It is important to visually inspect a surface prior to ATP testing to confirm no visible residues. Visual inspection before swabbing can save on swab costs and corrective action time.
The recommended times for ATP testing are:
- Test after cleaning the environment to provide valuable information related to removing filth and reducing risk.
- Test during vectoring to determine the root cause of elevated microbial counts.
While ATP testing cannot provide information about the types of contaminants present on a surface, it is a fast, low-cost, and effective verification of cleaning and sanitation.
When to do Microbial Testing?
The next layer of environmental monitoring is microbial testing. Contrary to ATP testing, microbial testing, which includes both indicator organism and pathogen testing, should occur at the filthiest time of the week or day. The dirtiest time is customarily mid-production or right before shutdown for cleaning and sanitation. Microbial testing should also target sites where microbial contaminants are more likely to be found, such as in rough surfaces and crevices.
Indicator Organism Testing
Indicator organism testing is used to detect and monitor food safety and quality risks and includes the detection of spoilage organisms. Spoilage organisms, such as yeasts and molds, can contaminate air vents and HVAC systems and enter manufacturing areas. This is a major reason for air sampling in food plants. Spoilage organisms, including yeasts and molds, lactobacilli, and other spore-forming bacteria, can also be found in food products. These contaminants can cause off-odors and flavors and may produce gas, leading to shortened shelf life and reduced product quality.
Indicator organisms are a monitoring tool employed to measure the possible presence of difficult-to-detect pathogenic organisms. Indicator organisms survive under similar nutrient, chemical, and physical conditions of related pathogens, so their presence can offer information about the risk of pathogens in the environment. Food manufacturers may perform these tests in-house or send the samples to a 3rd party lab for evaluation. However, 3rd party lab testing increases the time to test results and adds to the environmental monitoring budget—the more robust the program, the greater the cost. Therefore, bringing these tests in-house can provide faster results and alleviate the additional cost of the 3rd party lab.
Enterobacteriaceae is the most recommended microbial indicator for usage. This bacterial group includes coliforms, E. coli, and other gram-negative organisms, including pathogens like Salmonella. These are heat-sensitive bacteria, so when found in post-heat processing areas, it indicates a breakdown in the controls to prevent post-process contamination.
Aerobic count (AC) and aerobic plate count (APC) provide a generic count of the total number of bacteria in a sample. Manufacturers frequently employ AC and APC because of the advantages of quick results and lower costs. Aerobic bacteria can lead to shortened shelf life and off-odors. Aerobe testing does not detect yeast, mold, Campylobacter, or other strictly anaerobic bacteria.
Food manufacturers may also test for indicator organism groups, such as staphylococci, pseudomonas, or enterococci, depending on their products and specific risks associated with those food items.
Times for initiating additional microbial indicator testing include:
- Following the discovery of a contaminant in a product sample.
- Following the observance of an increase in indicator organisms.
- Following an indication that there is a distinct possibility of microbial contaminants being present.
- Increased quality issues with shelf-life or spoilage
Pathogen Testing
In-house technicians collect pathogen test samples, and plants typically employ 3rd party laboratories to conduct listeria testing and salmonella tests for comprehensive bacteria detection. Other pathogens may be tested, such as Chronobacter sakazakii, in an infant food production environment if the product is susceptible to specific pathogens and the risk factors warrant additional testing. All types of Salmonella are pathogenic. Salmonella, linked to milk, poultry, eggs, flour, vegetables, spices, and nuts, is often found in low and high moisture areas, in areas with pest activity, and within air intakes.
Listeria monocytogenes is the only known pathogen within the Listeria genus. Therefore, authorities recommend only testing for Listeria spp. and assume it is Listeria monocytogenes. It is usually associated with cheese, raw vegetables, and ice. Listeria is typically located in cool or wet environments and drains.
Advantages of In-House Environmental Monitoring with Microbial Testing and ATP Testing
For microbial evaluations, indicator and spoilage organisms tested in-house provide faster results which are as accurate as 3rd party testing. There is a reduced risk associated with in-house testing because the plant can catch problems more quickly and immediately implement corrective actions. Sending samples to a 3rd party lab can delay discovering contaminants that enable pathogens to infect more products. In-house microbial testing does not require significant capital investments, and it is not necessary to hire lab technicians with advanced biology degrees.
Regarding ATP testing, rapid tests are available that provide results within seconds. They produce data in real-time and are relatively simple, effective, sensitive, and quantitative. In addition, ATP bioluminescence systems provide computerized data logging, portability, and visual readouts of ATP levels in Relative Light Units, of RLUs. In terms of cost, plants will see savings resulting from improvements in the cleaning with better quality, improved shelf life, and less time needed for managing the cleaning process and crew.
Conclusion
A robust Environmental Monitoring Program ultimately affects the overall safety and quality of manufactured products by efficiently applying ATP testing and indicator organism testing to drive a clean environment that will inhibit pathogen growth.
Charm Sciences fully understands the critical importance of having an effective environmental monitoring program. We use a philosophy of prevention while providing the tools to monitor facility sanitation and hygiene (ATP), spoilage organisms, and indicator organisms. Contact us today to learn how to employ a preventative strategy to protect your brands from premature spoilage and recalls using environmental sampling in conjunction with microbial indicator testing.
About Charm Sciences
Established in 1978 in Greater Boston, Charm Sciences helps protect consumers, manufacturers, and global brands from a variety of issues through the development of food safety, water quality, and environmental diagnostics tests and equipment. Selling directly and through its network of distributors, Charm’s products serve the dairy, feed and grain, food and beverage, water, healthcare, environmental, and industrial markets in more than 100 countries around the globe.