Peel Plate Microbial Tests
Microbial Testing Reinvented
Peel Plate Microbial Tests are advanced alternatives for indicator organism testing. Third-party validations confirm that our method is reliable: NCIMS, AOAC, and MicroVal.
Indicator Organism Tests Available:
Aerobic Count (Peel Plate AC), coliform (Peel Plate CC), Enterobacteriaceae (Peel Plate EB), E. coli/coliform (Peel Plate EC), heterotrophs (Peel Plate HET), Staphylococcus aureus (Peel Plate SA), yeast (Peel Plate Y), and yeast and mold (Peel Plate YM).
Benefits of Charm Microbial Peel Plate Tests

- Ready-to-use. Self-wicking; no spreading device needed.
- Colonies easily transferred or picked for additional confirmation/isolation/classification.
- Improved air circulation with spacing between stacked, interlocked plates.
- Buffered media formulation for robust performance with acidic foods and neutralizing buffers.
- pH adjustments not required for most samples.
- 12-month room temperature stable in a re-sealable foil zip bag.*
- Date tracking and count analysis available with the Peel Plate Colony Counter
*High Volume tests and certified milk labs require refrigerated storage (0 – 4.5°C).
View Our Microbial Testing Products
A wide range of Charm Peel Plates provide an advanced approach to your indicator organism testing with consistent, reliable results.
Peel Plate AC Microbial Test (Aerobic Count)
![]() Peel Plate AC nutrient rich non-selective media encourages the growth of aerobic, oxygen consuming bacteria. Aerobes appear as red colonies within 48 hours at 32 or 35ºC; size may vary. This test can also be incubated under anaerobic conditions to detect the presence of LAB bacteria.
Peel Plate AC nutrient rich non-selective media encourages the growth of aerobic, oxygen consuming bacteria. Aerobes appear as red colonies within 48 hours at 32 or 35ºC; size may vary. This test can also be incubated under anaerobic conditions to detect the presence of LAB bacteria.
Peel Plate CC Microbial Test (Coliform Count)
![]() Total Coliform Peel Plate CC selective media encourages the growth of coliform bacteria. The presence of coliform is an indicator of unsanitary conditions in raw products. Coliform are killed by effective heat steps in food processing. Coliform appear as red colonies within 24 hours at 32 or 35°C. Coliforms are closely regulated in dairy and some foods the US and Peel Plate Coliform tests are listed in the PMO.
Total Coliform Peel Plate CC selective media encourages the growth of coliform bacteria. The presence of coliform is an indicator of unsanitary conditions in raw products. Coliform are killed by effective heat steps in food processing. Coliform appear as red colonies within 24 hours at 32 or 35°C. Coliforms are closely regulated in dairy and some foods the US and Peel Plate Coliform tests are listed in the PMO.
Peel Plate EB Microbial Test (Enterobacteriaceae Bacteria)
![]() The Enterobacteriaceae bacteria are a family of gram negative bacteria encompassing coliform, E. coli, Shigella, Salmonella, and Yersinia. These bacteria indicate unsanitary conditions in raw products and are killed by heat steps in food processing. EB testing is used as a post-production verification of process and pathogen risk used, for example, to test dry milk powder and infant formula. EB is regulated globally, similar to coliform/E.coli regulation in the US. Incubation 24 to 48 hours at 37°C produce multiple colored round growth spots that are all recorded as EB. High Volume plates are available.
The Enterobacteriaceae bacteria are a family of gram negative bacteria encompassing coliform, E. coli, Shigella, Salmonella, and Yersinia. These bacteria indicate unsanitary conditions in raw products and are killed by heat steps in food processing. EB testing is used as a post-production verification of process and pathogen risk used, for example, to test dry milk powder and infant formula. EB is regulated globally, similar to coliform/E.coli regulation in the US. Incubation 24 to 48 hours at 37°C produce multiple colored round growth spots that are all recorded as EB. High Volume plates are available.
Visit the Peel Plate EB test page to learn more.
Peel Plate EC Microbial Test (E.coli & Coliform)
![]() Generic E.coli, considered a fecal risk indicator, is a subset of coliform and are differentiated with the Peel Plate EC formulation. Peel Plate EC are AOAC-RI validated for a number of dairy products, meat rinses, water testing, and environmental sampling. Coliform appear as round red colonies and E. coli as round blue colonies within 24 hours at 35°C and the sum of red and blue are total coliform.
Generic E.coli, considered a fecal risk indicator, is a subset of coliform and are differentiated with the Peel Plate EC formulation. Peel Plate EC are AOAC-RI validated for a number of dairy products, meat rinses, water testing, and environmental sampling. Coliform appear as round red colonies and E. coli as round blue colonies within 24 hours at 35°C and the sum of red and blue are total coliform.
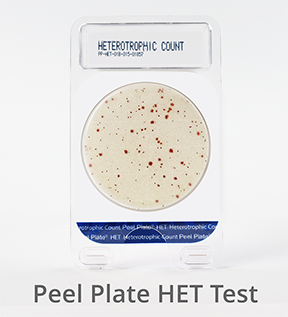
Peel Plate HET Microbial Test (Hetertrophic Count)
![]() Heterotrophs are aerobic bacteria that are more associated with water and nutrient-depleted matrices. Peel Plate HET nutrient-depleted media encourages the growth of heterotrophic organisms in water. Incubation at 20 to 28°C produces red growth spots within 5 to 7 days. Heterotrophs are used as indicators to assess biofilm build-up, and nutrient leaching in water distribution systems of food plants and water municipalities.
Heterotrophs are aerobic bacteria that are more associated with water and nutrient-depleted matrices. Peel Plate HET nutrient-depleted media encourages the growth of heterotrophic organisms in water. Incubation at 20 to 28°C produces red growth spots within 5 to 7 days. Heterotrophs are used as indicators to assess biofilm build-up, and nutrient leaching in water distribution systems of food plants and water municipalities.
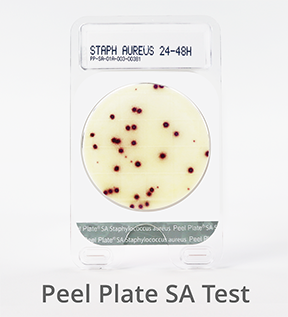
Peel Plate SA Microbial Test (Staphylococcus aureus)
Staphylococcus aureus can be present in eggs, meat, poultry, milk, and dairy products and are spread through hand contact with environmental surfaces or hand-prepared food items. Peel Plate SA tests are used to test finished products and environmental samples.
S. aureus bacteria appear within 24 hours, with an additional 24 hours for presumptive positive confirmation (48 hours total) at 35 to 37°C. Tests with no growth at 24 hours are considered negative for S. aureus.
Visit the Peel Plate SA test page to learn more.
Peel Plate Y Microbial Test (Yeast)
Peel Plate Y uses alternative indicators from the YM test to select for yeast colony growth only. It recommends 32°C incubation that is more favorable to yeast growth compared to mold. The method is particularly for use in foods naturally containing molds, i.e., blue cheese. It uses media containing selective agents and dyes to produce dehydrogenase enzymes, which react with a redox indicator to produce red or pink colored colonies.
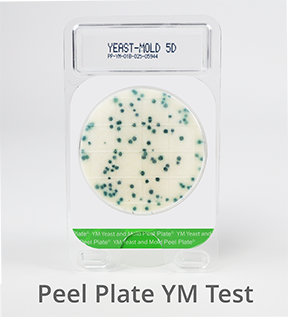
Peel Plate YM Microbial Test (Yeast & Mold)
![]() Peel Plate selective media with antibiotic prevents the growth of bacteria, and encourages the growth of fungi. Fungi organisms are primarily a food spoilage risk but can also produce toxins. Peel Plate YM are AOAC-RI validated for a variety of dairy products, fresh fruits and vegetables, juices, RTE foods, and environmental surfaces. Incubation 3 to 5 days at 25ºC produce green/blue growth spots. High Volume plates are available.
Peel Plate selective media with antibiotic prevents the growth of bacteria, and encourages the growth of fungi. Fungi organisms are primarily a food spoilage risk but can also produce toxins. Peel Plate YM are AOAC-RI validated for a variety of dairy products, fresh fruits and vegetables, juices, RTE foods, and environmental surfaces. Incubation 3 to 5 days at 25ºC produce green/blue growth spots. High Volume plates are available.
Industries
Environmental Monitoring
Certifications
Use microbial testing solutions you can trust. Third-party validations confirm that our method is proven reliable.
- NCIMS
- AOAC
- MicroVal
- ISO 9001:2015
How to Use Peel Plate Microbial Tests

Peel
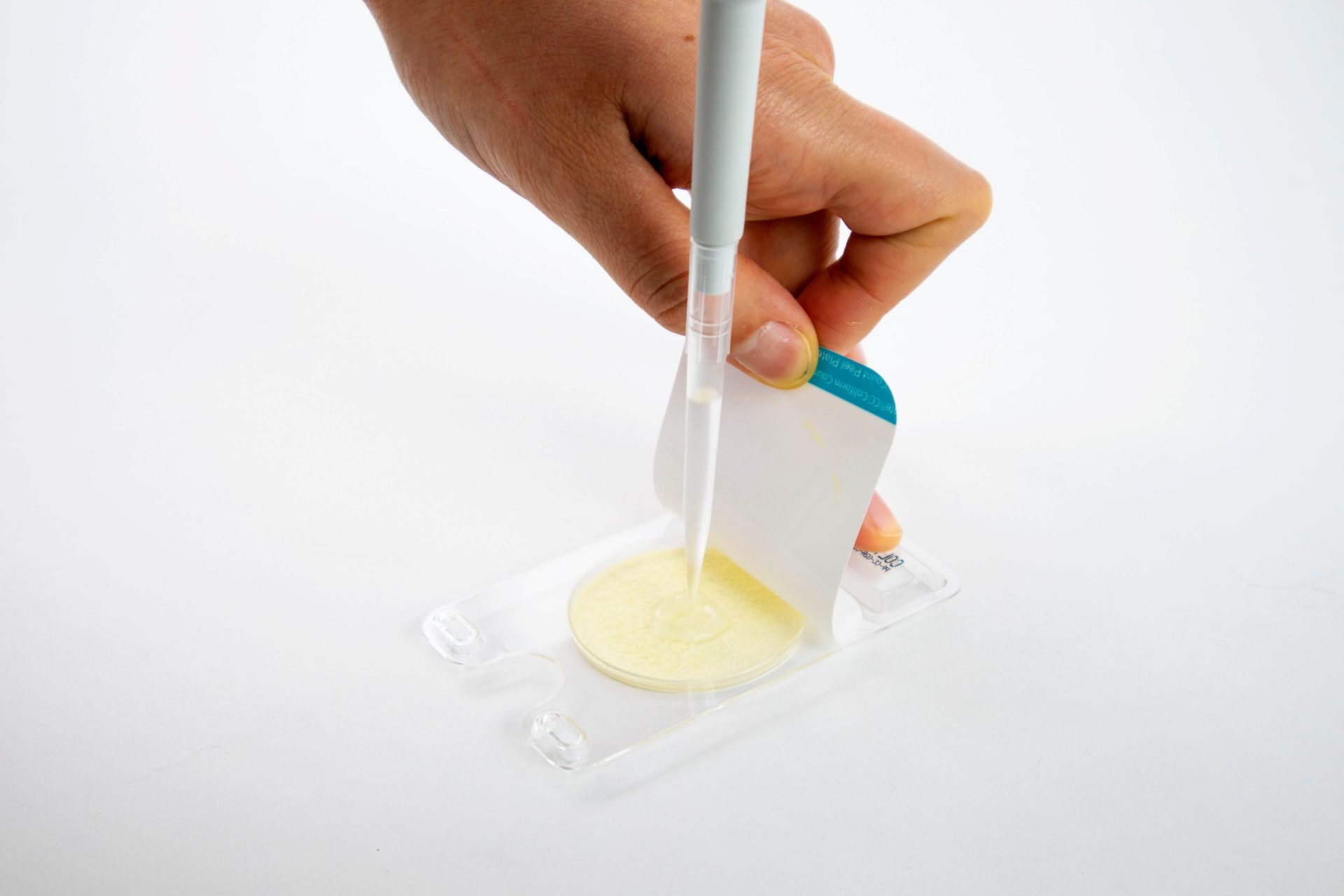
Pipet

Seal

Incubate

Fast & Accurate Colony Counting
The Peel Plate Colony Counters accurately count the colonies in under four seconds.
Contact Sales
Have a sales question? Send us a message and a sales representative will contact you.
By submitting your information via this form, you give Charm Sciences permission to contact you via email about updates, news, or offers that may interest you. It is Charm Sciences’ policy not to share any personally-identifying material obtained through our websites with any third party.