Peel Plate® EB Microbial Test
Quick, Accurate, and Easy-to-Understand Results for Onsite Testing
Enterobacteriaceae (EB) are a broad family of gram negative bacteria that are considered indicators for closely related pathogens like salmonella and cronobacter sakasakii that have caused food recalls and illnesses. With the Peel Plate EB test, you can trust that your test results are not only accurate, but also a reliable measure to ensure your compliance with industry-specific regulations. The Peel Plate EB test is simple to use, sensitive and provides fast, easy-to-read results. Innovations at every step in the testing process include self-wicking media that removes the need for a spreader device and speeds up the plating process, and the use of color changes to indicate bacterial growth, eliminating the subjective judgment required by alternative testing methods. The simplicity of The Peel Plate EB Microbial Test allows for testing to be brought into the plant without requiring an onsite microbiologist, resulting in precise data obtained while simultaneously cutting down on time and costs.
The Peel Plate EB test is internationally validated by MicroVal and has AOAC OMA first action status validated for use with specific food types. By using a test that is tailored to your industry, you can be sure that your results are in line with industry requirements and regulations.
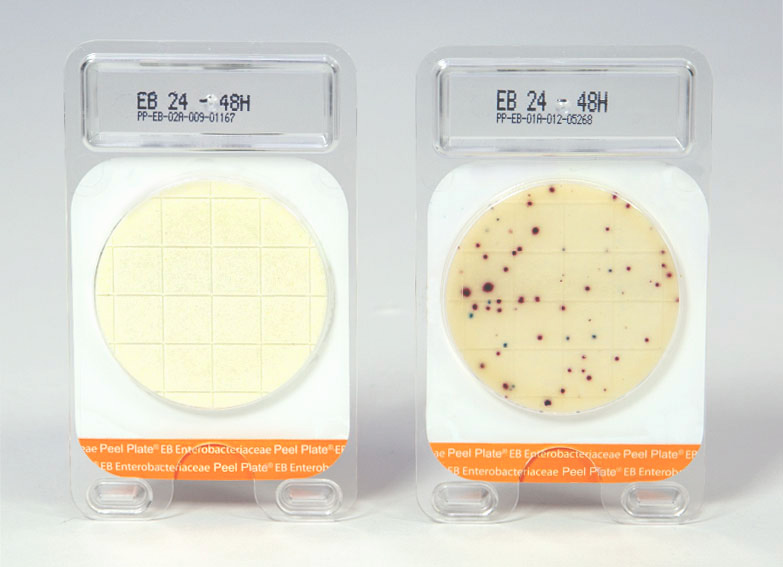

Use Descriptions
The Peel Plate EB Microbial Test plate contains prepared media in a shallow dish with an adhesive top. To use, simply peel back the cover, pipette the test sample in the middle of the plate (no spreading device is needed), close the cover, and incubate the plates at 37° C for 24 and up to 48 hours. Up to 20 plates can be stacked for space-efficient incubation.
High Volume (HV) plates are available to achieve high sensitivity in environmental samples and for sensitivity targets of 1 colony forming unit per gram (CFU/g) of food products that require dilution for testing.
Cultured Dairy (CD) plates are available to address interferences associated with cheese enzymes and lactobacillus cultures in for example yogurt, sour cream and cottage cheese.
Certifications
- OMA, AOAC 2018.05
- MicroVal 2017LR69
Use Description
The Peel Plate EB Microbial Test plate contains prepared media in a shallow dish with an adhesive top. To use, simply peel back the cover, pipette the test sample in the middle of the plate (no spreading device is needed), close the cover, and incubate the plates at 37° C for 24 to 48 hours. Up to 20 plates can be stacked for space-efficient incubation.
Contact Sales
Have a sales question? Send us a message and a sales representative will contact you.
By submitting your information via this form, you give Charm Sciences permission to contact you via email about updates, news, or offers that may interest you. It is Charm Sciences’ policy not to share any personally-identifying material obtained through our websites with any third party.

