Blog
Sanitation Verification: Food Production and Food Safety

This blog post is a 33 minute read
- Sanitation programs with frequent verification in food plants are the foundation to microbiological or allergen hazard control.
- Responsible individuals perform visual, olfactory, and adenosine triphosphate (ATP) tests that verify biologic cleanliness in proactive sanitation verification practices.
- Focusing on prevention practices is cost-effective and less disruptive to operations as opposed to reacting to hazard detection and the triggered corrective actions associated with control system failure.
- Complying with new food safety regulations requires education, training, and retraining for implementation of critical food safety principles.
The Food Safety Modernization Act (FSMA) and Preventative Controls for Human Foods (PCHF) regulations require food production facilities to perform hazard risk analysis and develop preventative controls that are described in a written hazard analysis risk-based preventative control (HARPC) plan located on-site. These rules have a phased implementation shown in tables below.
[wpdatatable id=60]

FSMA Finl Rules: Key Dates
The purpose of these rules is to produce safe foods for consumers using control processes that are validated and verified to reduce and eliminate microbiological, chemical, and physical hazards associated with food production and their raw ingredients.
It may seem like a daunting task for Preventative Controls Qualified Individuals (PCQI) to develop and implement the plan, but the assignment is within reach if there is a verifiable and effective sanitation foundation in their plant. Here we address some of the sanitation programs that will lead to food safety success and customer satisfaction.
Risk Analysis
For food production plants producing ready-to-eat (RTE) products and products with short shelf life, microbial contamination presents strong risks for pathogens, e.g. health risk and spoilage organisms. Pathogens such as hemorrhagic E. coli, salmonella, listeria and campylobacter can cause foodborne illnesses and are a leading cause of food recalls. Spoilage organisms such as molds can spoil food before use dates, upset consumers, and cause consumers to source different brands. Ingredients carrying allergenic proteins, such as milk, eggs, peanuts, tree nuts, fish, mollusks, and wheat (gluten) are another leading cause of food recalls when they are improperly labeled or cross-contaminated during food production operations. Huge financial loss, damage to company brand and reputation, jail sentences, bankruptcy, and death from associated illness are all possible consequences to companies that experience food safety recalls. Preventative control processes must be in place to reduce these risks.
Prerequisites Needed by Those Responsible
The PQCI on-site has the responsibility for developing, implementing, and presenting the HARPC during audits and inspections. Previous blogs have discussed sanitation as a prerequisite to FSMA. Sanitation controls are easily verified and documented regularly and are the foundation in a documented microbial or allergen cross-contamination control prevention plan. The sanitation prerequisite includes personnel hygiene, food contact surface cleaning, surrounding area cleaning, training, as well as extension of sanitation to raw source supplies such as the farm or other material suppliers. Sanitation is the underlying foundation to preventative control that the PQCI and food safety consultants need to implement.

Swabbing using a PocketSwab ATP swab
Preventative Control Pyramid
The base to the preventative control pyramid is basic Good Manufacturing Procedures, cleaning and sanitizing, and performing visual and smell (olfactory) assessments of the food process surfaces (Zone 1) and perimeter (Zones 2 and 3). Visual and olfactory assessments of the production area are fast and inexpensive verifications performed by an empowered sanitation crew that typically work the 3rdshift.
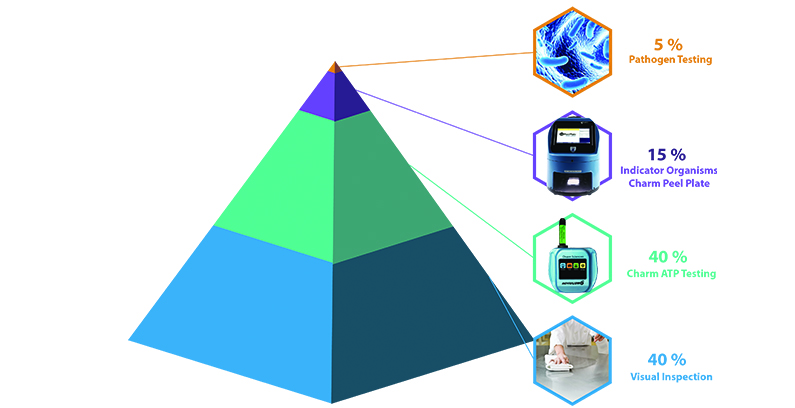
Preventative Control Pyramid
How ATP Systems Help
In addition to the most basic visual and olfactory assessments, many food production facilities use ATP tests that verify sanitation at a higher level detecting biological residuals still present but that are not visible. These residues can become the growth nutrients and attachment sites for harboring microbial hazards, or in the case of an allergenic protein, cross-contamination to other foods produced. ATP sanitation verification has multiple values in that it further enhances beyond visual biological dirt detection and serves as a training tool for proper cleaning procedures and improvement. For example, sanitization without cleaning can cause a build-up of biofilm that protects microbes from sanitizer activity. Finding ATP on these areas teaches the importance of soap and elbow grease in the cleaning process and how it is more effective than just a superficial spray with a disinfectant.
When ATP is detected, respond by recleaning and retesting, cordoning off positive areas from traffic, and vectoring by performing a spider web type search for other impacted areas. These steps all effectively make a preemptive strike against microorganisms and the niches in which they hide. While ATP detection cannot be correlated directly to microbial growth or concentration, reacting to a population of ATP positives locations and reducing them 10-fold with remediation has the result of reducing the population of microbial positives a similar 10-fold. This is because the ATP is detecting the precursors to microbial growth and attachment as well as the locations and biofilms in which microbes thrive.
Quickly identifying procedural breakdowns in sanitation acts as an early warning of future problems to investigate and correct with minimal downtime to production. As a food production facility Plant Manager explained, “we treat ATP is the early warning indicator to the smell “smoke” before the “fire” break out (pathogens) in the plant.” ATP systems also serve an important FSMA documentation requirement providing evidence of date, time, result, and corrective actions in sanitation operations.
Sensitivity and speed of an ATP system are very important. Some systems work in just 5 seconds allowing sanitation crews to quickly assess their areas and move on to the next. The more sensitive, the less food residue and microbes are present. Some ATP systems offer enhanced sensitivity swabs creating a two-step verification process; one is for routine, between-shift cleaning and one is for allergen residues when different products are packaged on a shared production line.
Microbial Reduction Verification
Microbial indicator testing is another microbe log reduction verification option in the control pyramid. A food plant might periodically perform an aerobic count or an Enterobacteriaceae test in the food process areas to find and correct likely harborage and survival locations for their microbial pathogen cousins. For example, 100 cfu/mL aerobic count is considered a minimum control level for listeria control while enterobacteriaceae <1 or 10 cfu/mL are indicators that related microbes like E. coli or salmonella could be surviving the cleaning process.
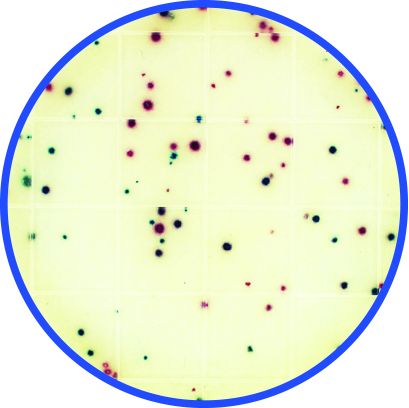
Peel Plate EB test
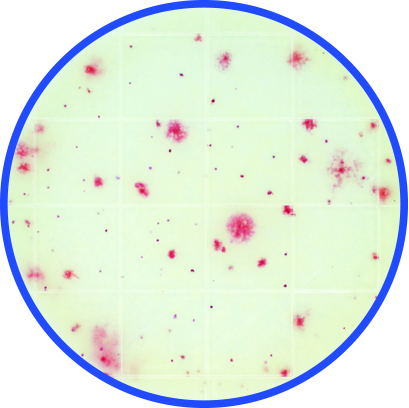
Peel Plate AC test
These indicator tests are faster and easier to perform than conventional pathogen tests and are more acceptable for in-plant testing allowing more rapid response. Additionally, this data may be collected and remediated to demonstrate process control before any pathogen tests samples are taken. Indicator testing serves the purpose of demonstrating the suitability for purpose of the real-time visual-clean and ATP systems be used, providing additional evidence that the real-time systems are detecting and controlling problematic cleaning areas and that the microbial levels in those areas meet cleaning specifications.
The sanitation verification program should actively search and remediate positive locations, vector from positive sites, and seek corrective action for positive trends. Indicator testing can serve as verification of real-time corrective action suitability for purpose. Such a program helps the PCQI develop a successful real-time plant management tool that is within their control and that minimizes the risk of failure.
Validate the System is in Control
The final pinnacle of the control pyramid is the “validation of no pathogens or allergens in food or production area”. This is achieved with the preventative foundation of real-time measures performed by a trained sanitation crew: 1) Visual-olfactory clean verification and 2) ATP clean verification before food contact. Areas are reprocessed and corrected based on these preliminary measurements which are fast and easy to perform. Periodic indicator testing verifies the real-time measures are effective. These foundations should be in place before pathogen tests are performed.
Whether controlling microbiological or allergen food cross-contact risks, the base of the control pyramid is 95% prevention with documented verification testing and the final 5% the resulting validation test that preventative controls are in place and effective. Focusing on prevention practices is more cost-effective and less disrupting to operations than reacting to hazard detection and the triggered corrective actions associated with control system failure.
Connecting the Dots
FSMA and PCHF require education, training, and retraining for implementation of these important food safety control practices. Charm Sciences Inc. assists food plants in identifying their risks and developing validation and verification plans for compliance. Charm personnel train on-site and develop customer competencies for required documentation for verification and validation of preventative controls.
About Charm Sciences
Established in 1978 in Greater Boston, Charm Sciences helps protect consumers, manufacturers, and global brands from a variety of issues through the development of food safety, water quality, and environmental diagnostics tests and equipment. Selling directly and through its network of distributors, Charm’s products serve the dairy, feed and grain, food and beverage, water, healthcare, environmental, and industrial markets in more than 100 countries around the globe.


